Energy, Enzymes, and Catalysis Problem Set
Problem 16 Tutorial: Energy requiring and energy yielding reactions
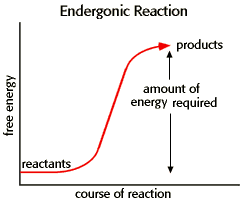
Endergonic Reaction
| An energy diagram for an endergonic or nonspontaneous reaction is shown to the right. The energy level of the products is higher than the energy level of the reactants. Energy must be added for this reaction to proceed. The amount of energy required is termed ΔG, which is greater than zero. The equilibrium constant for an endergonic reaction is less than 1. |
|
Department of Biochemistry and Molecular Biophysics
University of Arizona
Revised: October 2004
Contact the Development Team
http://biology.arizona.edu
All contents copyright © 1996. All rights reserved.





