The Biology Project > Biochemistry > The Chemistry of Amino Acids
Basic Amino Acids
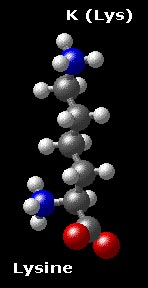
Basic amino acids are polar and positively charged at pH values below their pKa's, and are very hydrophilic. Even though the basic amino acids are almost always in contact with the solvent, the side chain of lysine has a marked hydrocarbon character, so it is often found NEAR the surface, with the amino group of the side chain in contact with solvent. Note that in the drawing, histidine is shown in the protonated form, while at pH 7.0, the imidazole would exist predominantly in the neutral form.





The Biology Project > Biochemistry > The Chemistry of Amino Acids
http://biology.arizona.edu
All contents copyright © 2003. All rights reserved.