Energy, Enzymes, and Catalysis Problem Set
Problem 14 Tutorial: Interpreting an "S-shaped" enzyme kinetics curve
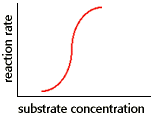
|
The graph shows the results of reaction rate vs substrate concentration for a(n): |
Allosteric Enzymes
Enzymes with multiple subunits have quaternary structure. One consequence of multiple subunits is that individual catalytic subunits each have their own active site. This means that an enzyme with quaternary structure can bind more than one substrate molecule. Allostery means "different shape." Allosteric enzymes change shape between active and inactive shapes as a result of the binding of substrates at the active site, and of regulatory molecules at other sites. In the simple case of an allosteric enzyme with an active and inactive form, the change in reaction rate with increasing substrate concentration is typically an "S-shaped" curve.
The meaning of the S-shaped curve for allosteric enzyme
At low substrate concentration, the enzyme is in the inactive shape (inactive conformation). As the substrate concentration is increased, substrate binds to enzyme and triggers a conformation change to the active shape of the enzyme. After the first substrate is bound, the second and subsequent substrates all bind more readily. This is termed "cooperativity," and the S-shaped curve indicates cooperative binding of substrate. The result of the change to the active shape is a steep increase in both substrate binding, and as a result, in reaction rate over a narrow range of substrate concentration. When all active sites on the allosteric enzyme are occupied with substrate, a plateau is reached.
The importance of allosteric enzymes to cellular regulation
Allosteric enzymes can be regulated between very low and very high reaction rates with only small changes in substrate concentration. Allosteric enzymes are used by cells to regulate metabolic pathways where the concentration of cellular substrates fluctuate over narrow concentration ranges.
Department of Biochemistry and Molecular Biophysics
University of Arizona
Revised: October 2004
Contact the Development Team
http://biology.arizona.edu
All contents copyright © 1996. All rights reserved.






